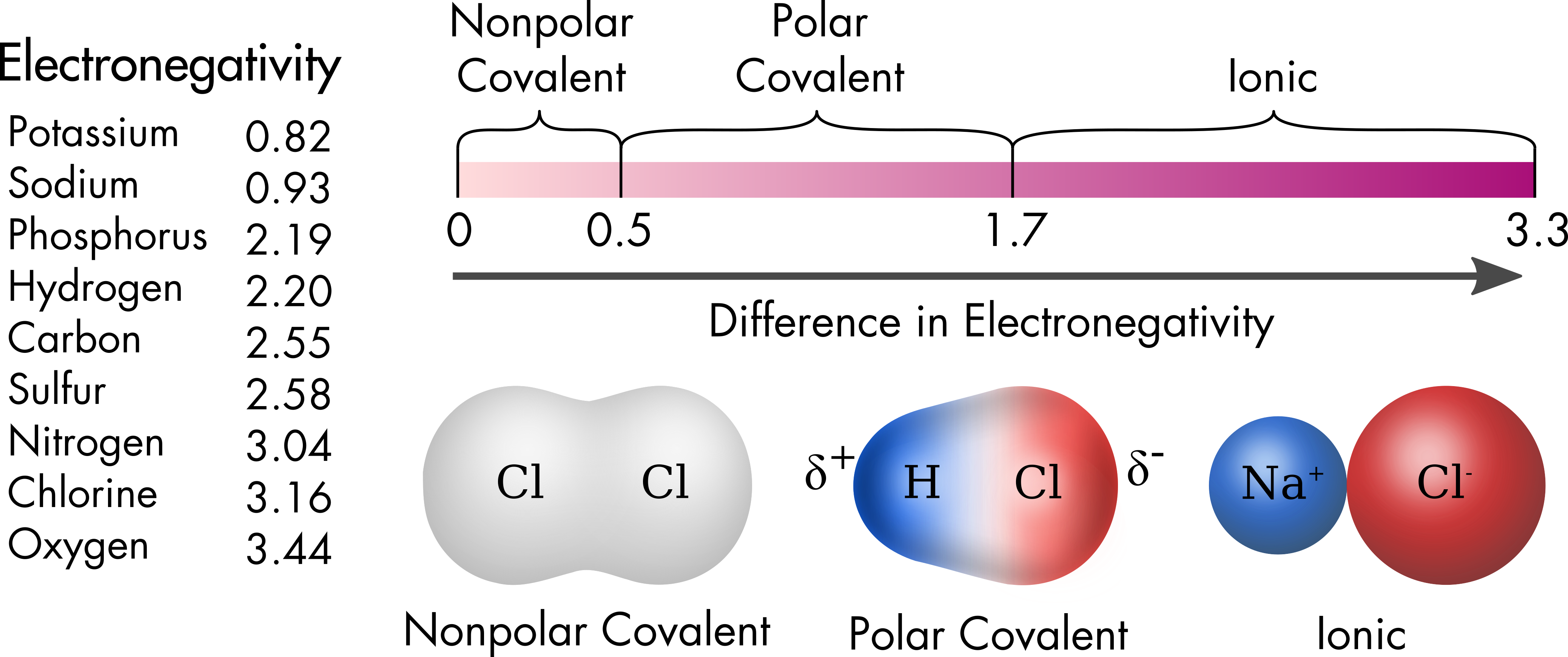
Carbon Chlorine Electronegativity . Electronegativity is used to predict. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. 119 rows values for electronegativity run from 0 to 4. The suggested values are all. what happens if two atoms of equal electronegativity bond together?
from neurotext.library.stonybrook.edu
The suggested values are all. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows values for electronegativity run from 0 to 4. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is used to predict. what happens if two atoms of equal electronegativity bond together? the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density.
Cellular Neurophysiology
Carbon Chlorine Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. what happens if two atoms of equal electronegativity bond together? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. The suggested values are all. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. Electronegativity is used to predict.
From es.dreamstime.com
Tabla Periódica Del Electronegativity Imagen de archivo Imagen 38153931 Carbon Chlorine Electronegativity what happens if two atoms of equal electronegativity bond together? electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. The suggested values are all. Electronegativity is used to predict. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. the. Carbon Chlorine Electronegativity.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Carbon Chlorine Electronegativity Electronegativity is used to predict. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. why does electronegativity increase across a period? what happens if two atoms of equal electronegativity bond together? provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in.. Carbon Chlorine Electronegativity.
From www.numerade.com
SOLVED PLEASE HELP 15 POINTS AND MARKED BRAINLIST... Use the chart to Carbon Chlorine Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. what happens if two atoms of equal electronegativity bond together? Electronegativity is used to predict. electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. why does. Carbon Chlorine Electronegativity.
From www.pinterest.co.uk
Illustration about Periodic table of elements with electronegativity Carbon Chlorine Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. what happens if two atoms of equal electronegativity bond together? 119 rows values for electronegativity run from 0 to 4. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair. Carbon Chlorine Electronegativity.
From www.nuclear-power.com
Carbon Electron Affinity Electronegativity Ionization Energy of Carbon Chlorine Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a. Carbon Chlorine Electronegativity.
From mungfali.com
Periodic Table Of Elements Electronegativity Carbon Chlorine Electronegativity what happens if two atoms of equal electronegativity bond together? Electronegativity is used to predict. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the. Carbon Chlorine Electronegativity.
From sciencenotes.org
List of Electronegativity Values of the Elements Carbon Chlorine Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict. The suggested values are all. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. electronegativity is. Carbon Chlorine Electronegativity.
From lessoncampuspolypide.z5.web.core.windows.net
Electronegativity And Polarity Chart Carbon Chlorine Electronegativity why does electronegativity increase across a period? provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. Electronegativity is used to predict. Consider. Carbon Chlorine Electronegativity.
From www.numerade.com
SOLVED Part 1 In compound (V) which coloured atom is the most Carbon Chlorine Electronegativity provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. what happens if two atoms of equal electronegativity bond together? The suggested values are all. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is used to predict. 119 rows. Carbon Chlorine Electronegativity.
From www.slideserve.com
PPT Ch. 16 Covalent Bonding PowerPoint Presentation, free download Carbon Chlorine Electronegativity Electronegativity is used to predict. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. what happens if two atoms of equal electronegativity bond together? The suggested values are all. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. electronegativity is. Carbon Chlorine Electronegativity.
From exoyzbldb.blob.core.windows.net
Is Chlorine Electronegative Or Electropositive at Otis King blog Carbon Chlorine Electronegativity The suggested values are all. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. why does electronegativity increase across a period? the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. electronegativity is a chemical property that. Carbon Chlorine Electronegativity.
From www.slideshare.net
Electronegativity part two Carbon Chlorine Electronegativity the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. The suggested values. Carbon Chlorine Electronegativity.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Carbon Chlorine Electronegativity Electronegativity is used to predict. what happens if two atoms of equal electronegativity bond together? 119 rows values for electronegativity run from 0 to 4. The suggested values are all. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. Consider sodium at the beginning of period 3. Carbon Chlorine Electronegativity.
From www.chemtopper.com
Video quiz on electronegativity part 4 on polarity in organic molecules Carbon Chlorine Electronegativity what happens if two atoms of equal electronegativity bond together? Electronegativity is used to predict. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The suggested. Carbon Chlorine Electronegativity.
From www.sliderbase.com
Pauling scale of electronegativity; Carbon Chlorine Electronegativity Electronegativity is used to predict. The suggested values are all. what happens if two atoms of equal electronegativity bond together? electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. provided. Carbon Chlorine Electronegativity.
From byjus.com
28. Electronegativity of carbon atoms? Sp2 hybridised or sp how to Carbon Chlorine Electronegativity 119 rows values for electronegativity run from 0 to 4. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. Electronegativity is used to predict. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. why does electronegativity. Carbon Chlorine Electronegativity.
From www.reddit.com
Electronegativity/ covalent and ionic bonds r/chemhelp Carbon Chlorine Electronegativity electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. provided that you understand what happens when electronegative atoms like fluorine or chlorine are attached to carbon atoms in. Electronegativity is used to predict. what happens if two atoms of equal electronegativity. Carbon Chlorine Electronegativity.
From slideplayer.com
C and C have the same electronegativity. ppt download Carbon Chlorine Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's. Electronegativity is used to predict. electronegativity is a chemical property that measures how likely an atom is to attract a shared pair. Carbon Chlorine Electronegativity.